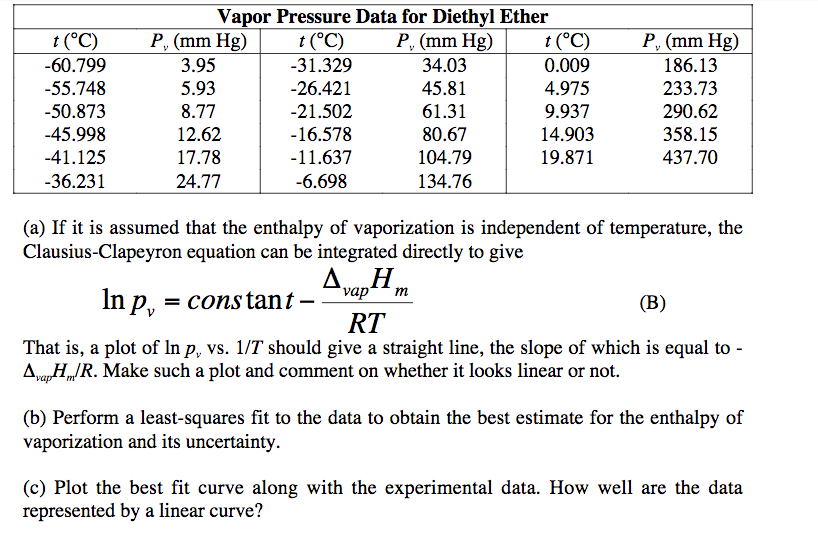
Diethyl Ether Vapor Pressure
34 5 deg c vp exp database.
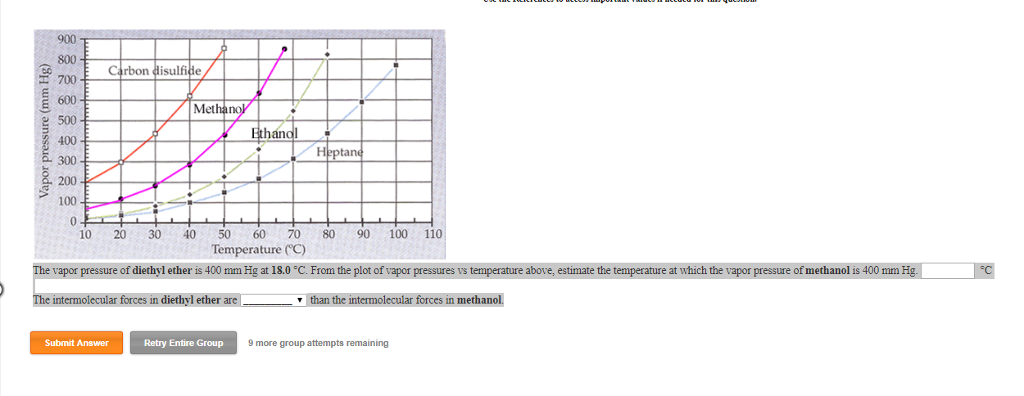
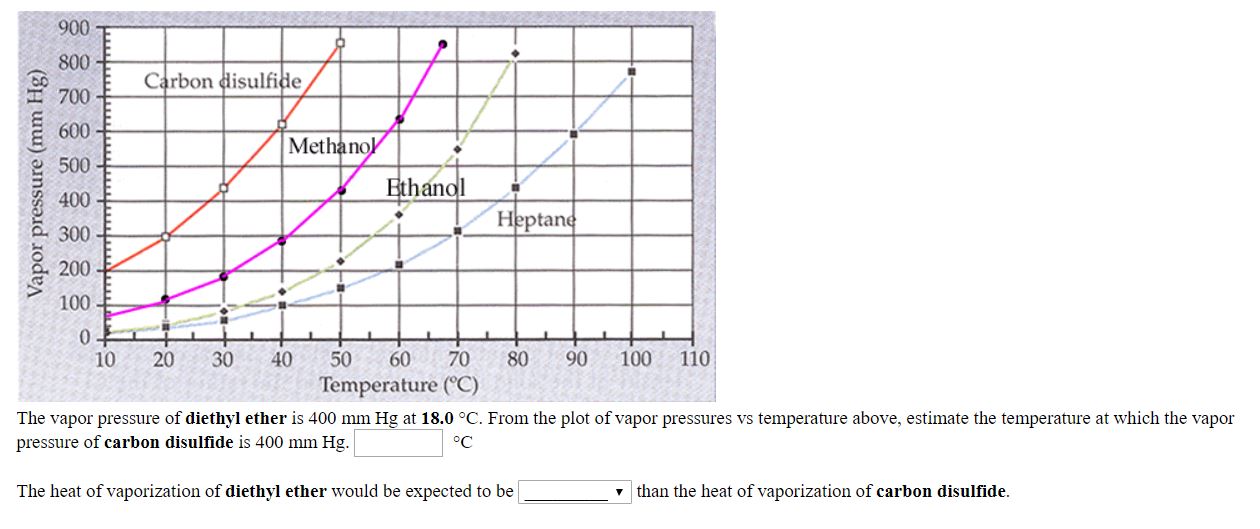
Diethyl ether vapor pressure. Diethyl ether or simply ether is an organic compound in the ether class with the formula c 2 h 5 2 o sometimes abbreviated as et 2 o see pseudoelement symbols it is a colorless highly volatile sweet smelling ethereal odour flammable liquid it is commonly used as a solvent in laboratories and as a starting fluid for some engines. Recall that diethyl ether has weak dispersion forces which meant that the liquid has a high vapor pressure. δh of vaporization. Temperature k a b c reference comment.
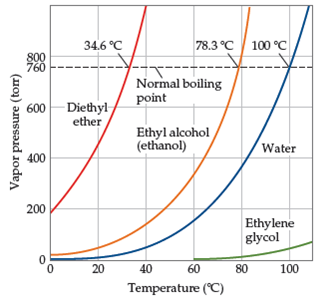
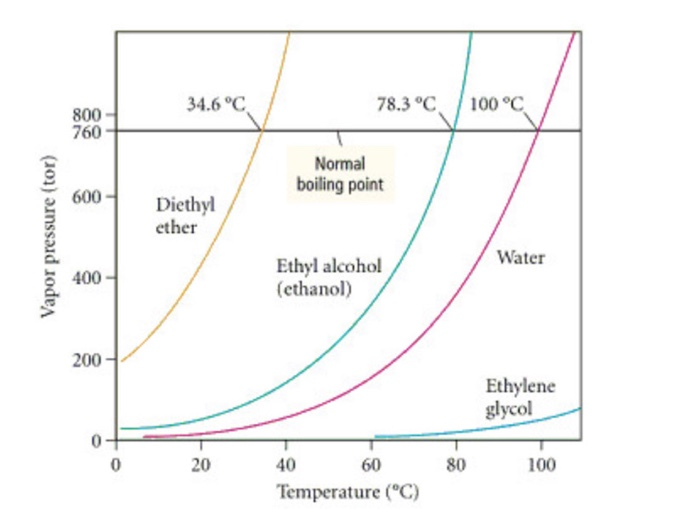
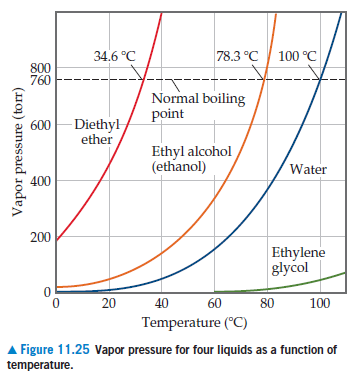
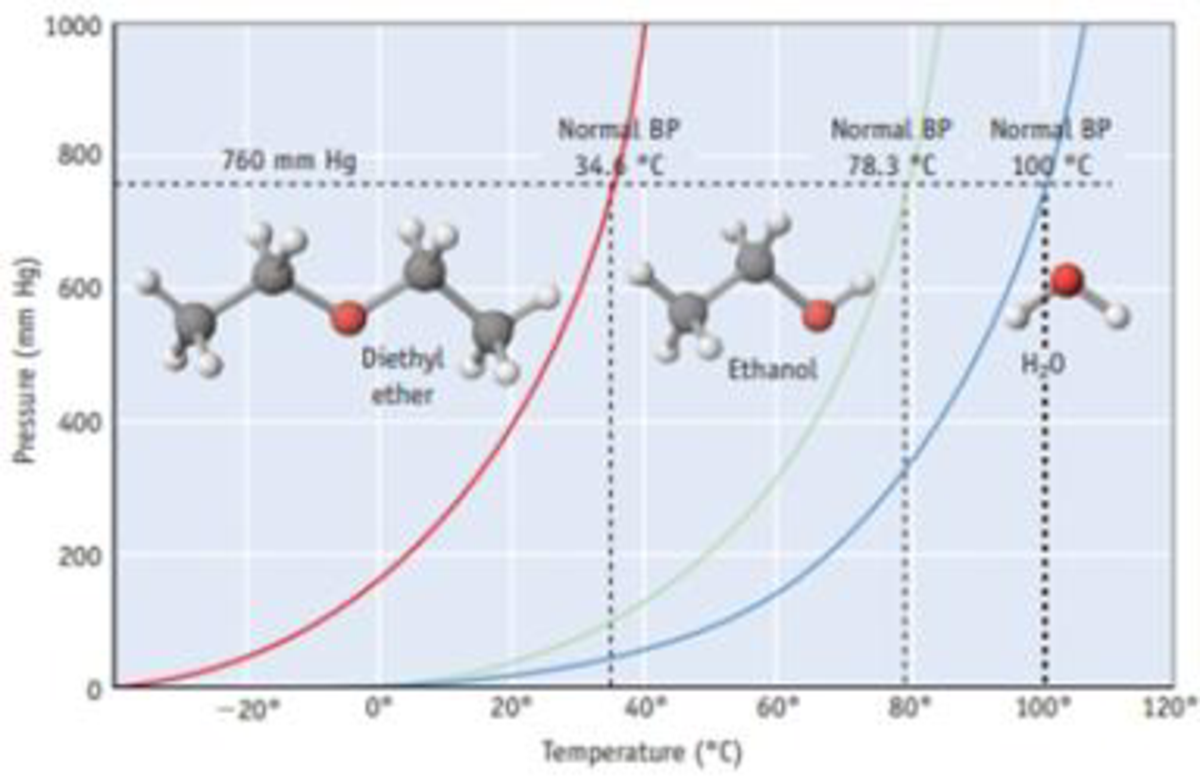
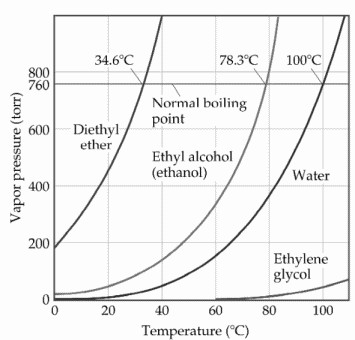
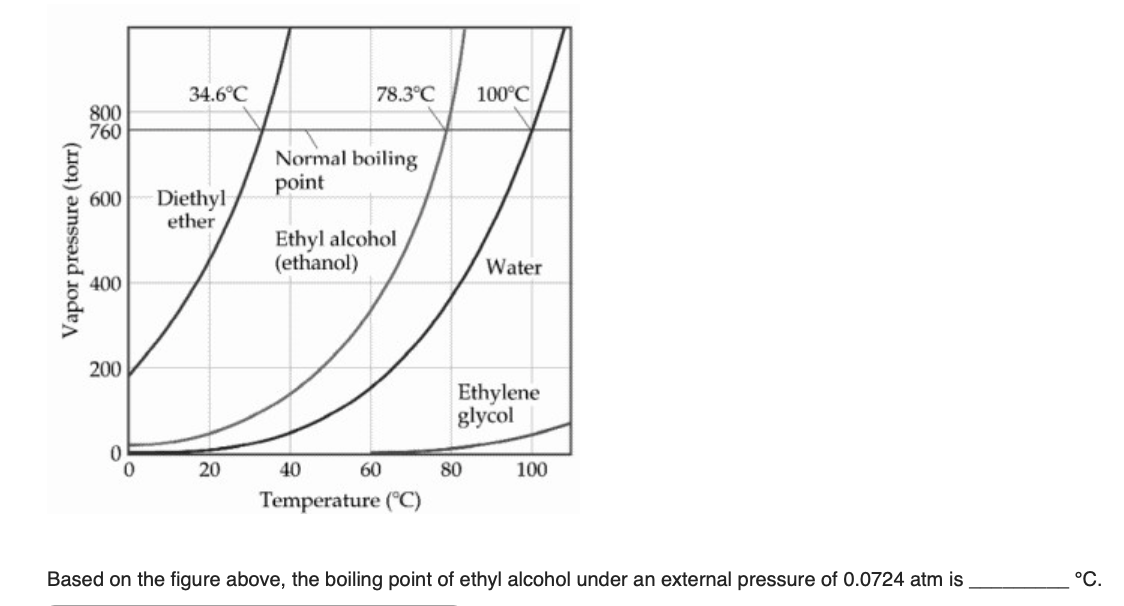
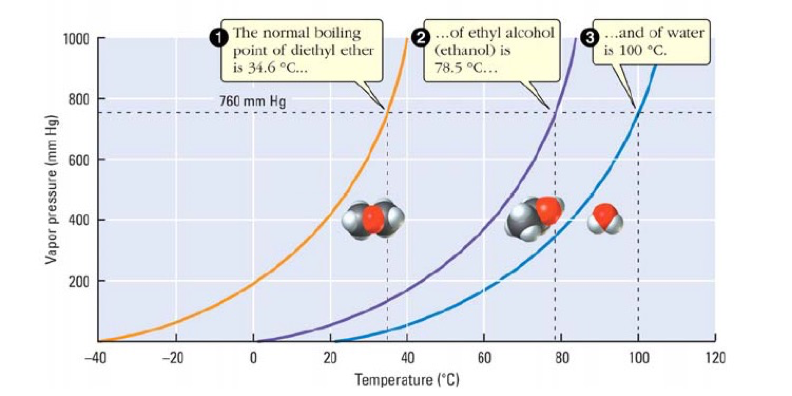
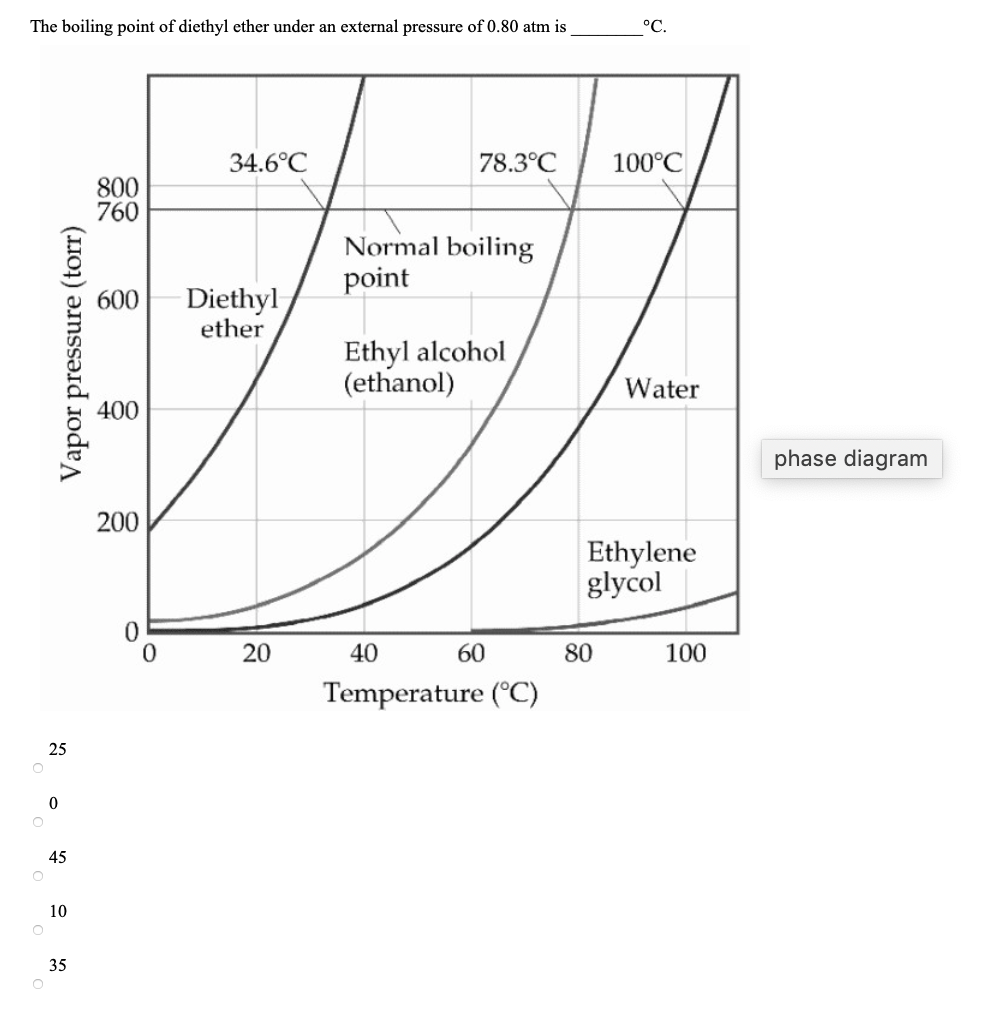
The vapor pressure of a liquid varies with its temperature as the following graph shows for water. 1995 boiling pt melting pt vapor pressure estimations mpbpwin v1 42. 1941 63 2267 2272. The line on the graph shows the boiling temperature for water.
116 3 deg c bp exp database. The weak forces also mean that it does not require a large an input of energy to make diethyl ether boil and so it has a relatively low normal boiling point of 34 6 c. Diethyl ether ch3ch2och2ch3 was one of the first chemicals used as an anesthetic. This difference can be demonstrated by means of a buret clamped upside down in a reservoir of water several ml of air trapped in the buret above a column of water and a funnel attached to the buret nozzle.
Ambrose sprake et al 1972. The handling of this chemical may incur notable safety precautions. Material safety data sheet. 47 02 adapted stein brown method melting pt deg c.
541 mean vp of antoine grain methods mp exp database. Torr and at 13 9 c it has a vapor pressure of 343 torr. At 34 6 c diethyl ether has a vapor pressure of 760. It was formerly used as a general anesthetic until.
Vapor pressure of diethyl ether. Coefficents calculated by nist from author s data. The experimental data shown in these pages are freely available and have been published already in the ddb explorer edition the data represent a small sub list of all available data in the dortmund data bank for more data or any further information please search the ddb or contact ddbst. Boiling pt deg c.
The volatility of diethyl ether is evident from a vapor pressure more than 20 times that of water at these temperatures. What is the δh of vaporization for diethyl ether. Vapor pressure at 25 o c. 101 15 mean or weighted mp vp mm hg 25 deg c.
The density of gaseous dimethyl ether j. 5 38e 02 mm hg at 25 deg c water solubility.