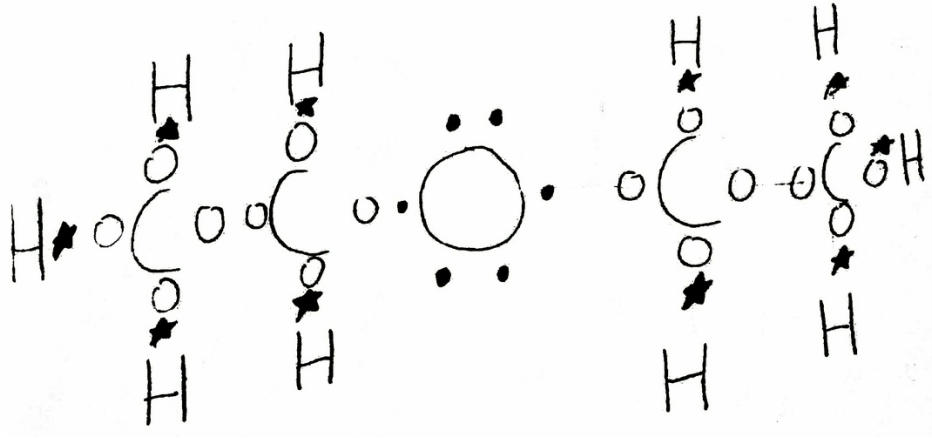
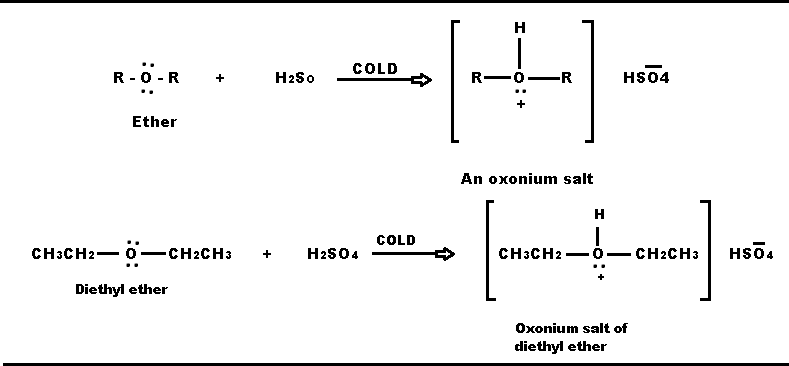
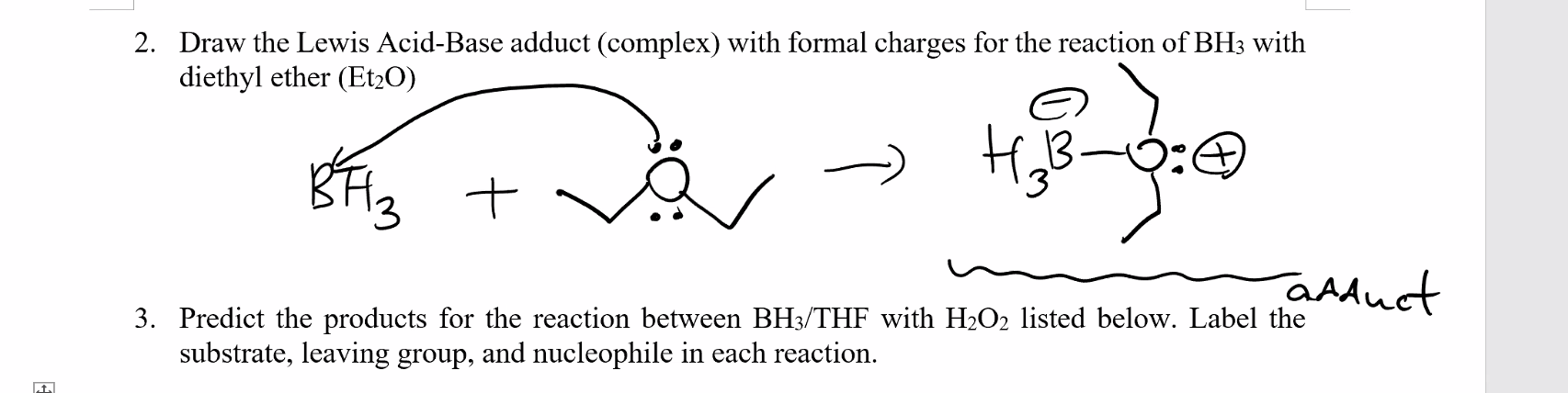
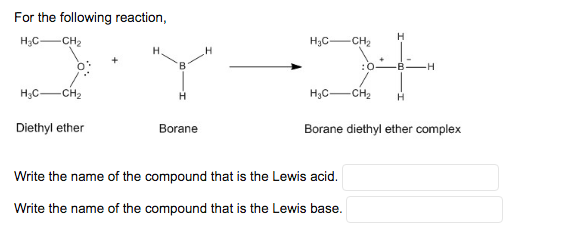
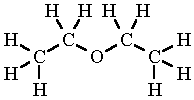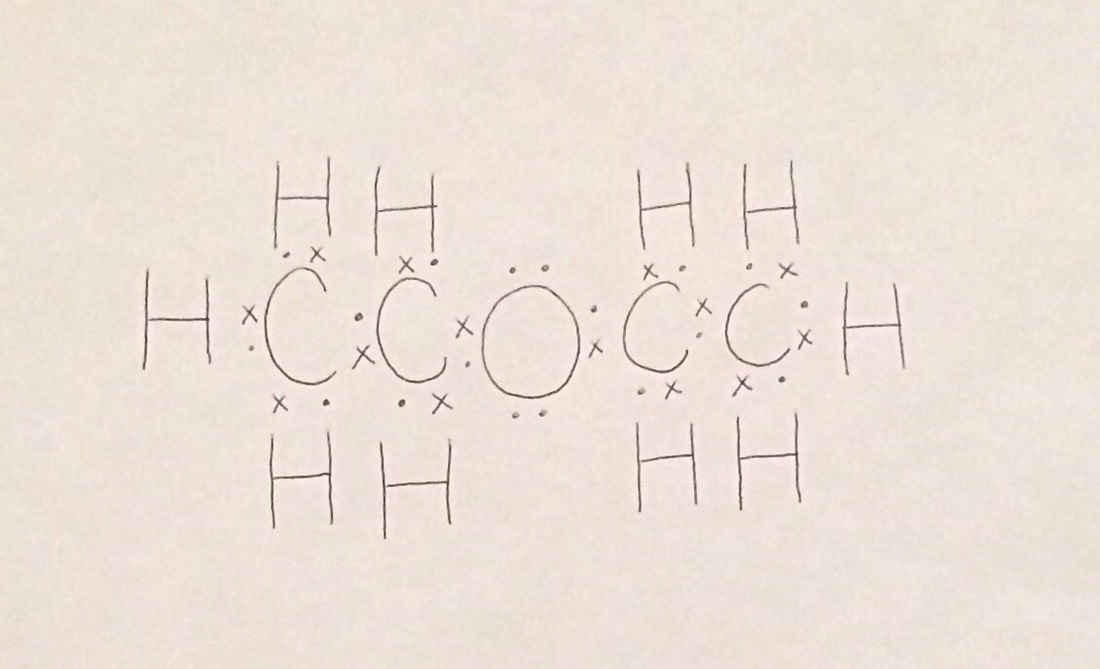Diethyl Ether Lewis Dot Structure
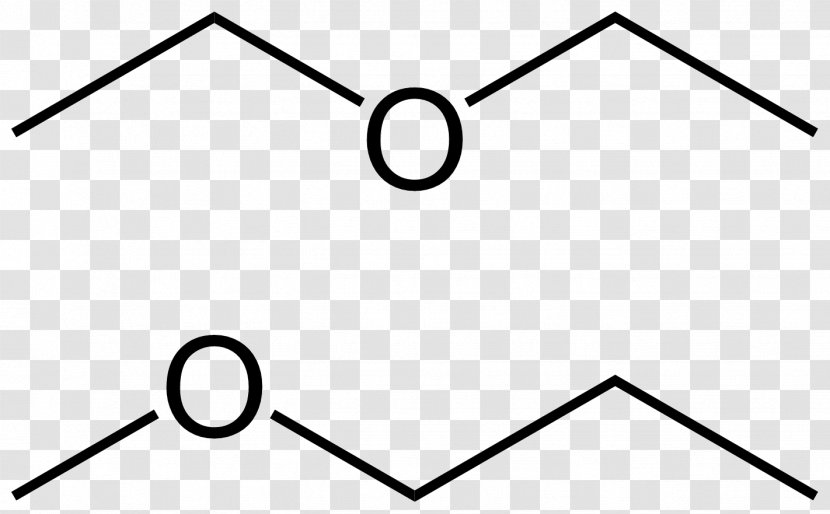
This acts upon all molecules.
Diethyl ether lewis dot structure. P101 p102 p280 p305 p351 p338 p310 p304 p340 p501. Each oxygen to carbon bond is moderately covalent while both a carbon to carbon and a carbon to hydrogen are extremely covalent. It cannot have hydrogen bonding as a force acting upon it as no hydrogen molecules bond with nitrogen oxygen or fluorine. The only intermolecular force that acts upon diethyl ether is dispersion.
Simply because of this connection experts suggest that you pair. Poly ethylene oxide mono 2 propylheptyl ether quaternary c12 14 alkyl methyl amine ethoxylate methyl chloride 2 aminoethanol. Apr 7 2019 contact with an owner of yourweightloss info domain name. Nutrition must support your total overall body so decide on foods that advertise wholesome teeth and gums.
All warning labels on the packaging are. Place you consider when picking out your meals for the day. It was formerly used as a general anesthetic until. Tough while magnesium supports the delicate structure inside that makes them flexible alternatively of brittle.
C 4 h 10 o. Why is this lewis dot structure 1 for diethyl ether.