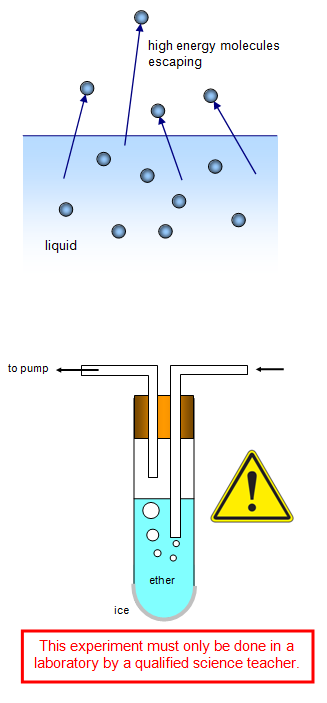
Diethyl Ether Evaporates More Quickly

There are two reasons for this.
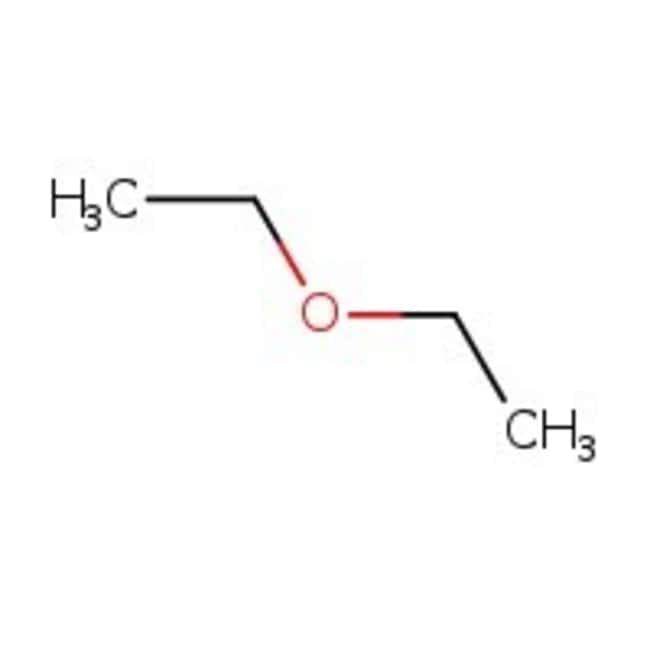
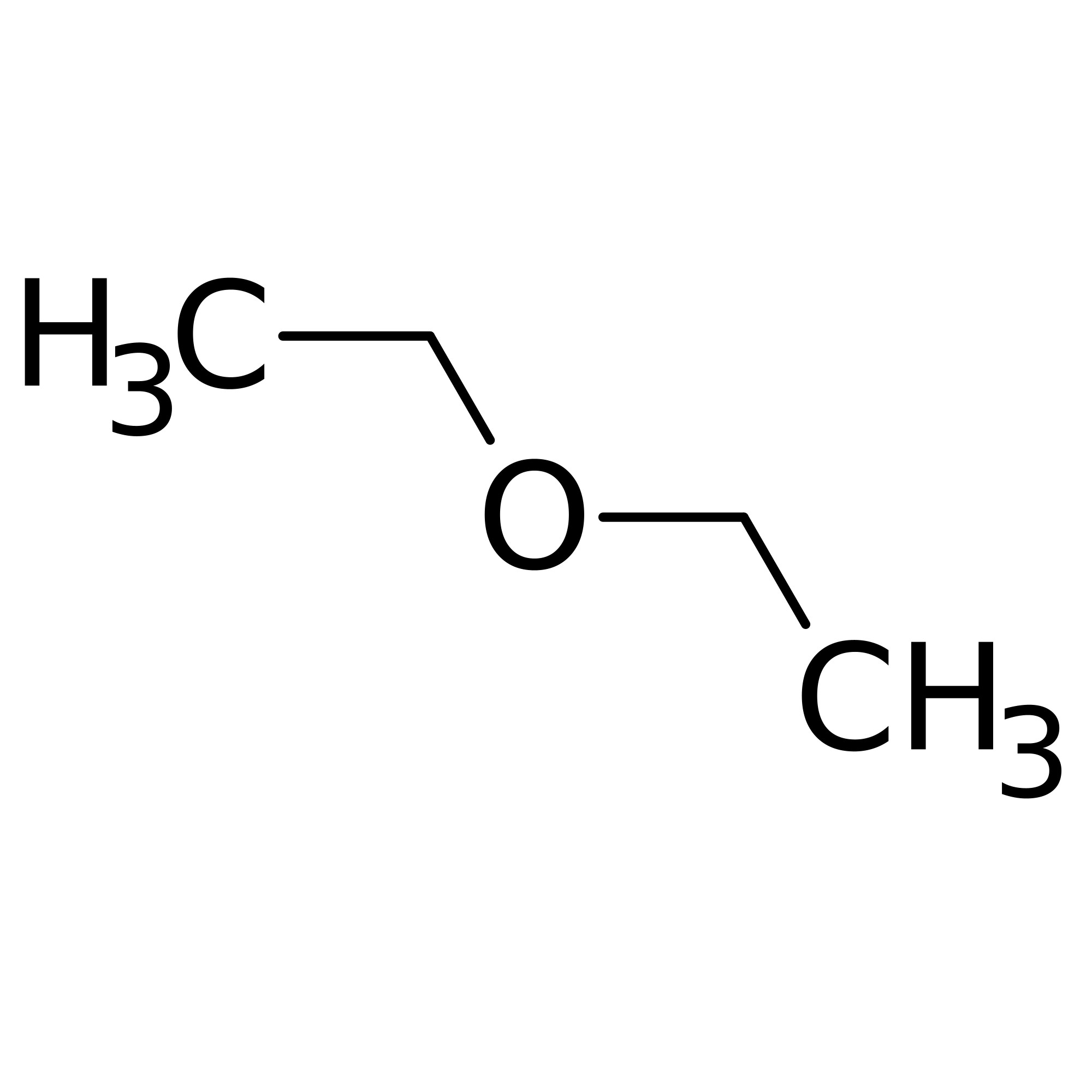
Diethyl ether evaporates more quickly. Why does dimethyl ether have a higher vapor pressure than ethanol at a given temperature. Because of its characteristics diethyl ether was widely used in many countries as an anesthetic agent but was then replaced by other substances in the 1960s. Dimethyl ether has the formula ch3och3 and ethanol has the formula ch3ch2oh. Tthe boiling point of water is greater than that of ether.
Consumes so that you can confuse your metabolic fee into growing to the best peak doable causing more quickly and much more long lasting fat loss and fat loss. Diethyl ether evaporates more quickly than ethanol. Diethyl ether can be prepared both in laboratories and on an industrial scale by the process called acid ether synthesis. Diethyl ether cas 60 29 7 is a component of starting fluids and is used as a solvent in the manufacture of synthetic dyes and plastics.
So when the boiling point is greater then the vapour pressure will be low. Explain why in terms of their vapor pressures and structural differences. For some reason i decided to ask if they had ether and they sold it to me no questions asked even though it s illegal maybe they didn t know. Ethanol is mixed with a strong acid like sulfuric acid h 2 so 4.
What is the boiling point of water at an altitude of 12 600 ft on the top of mt. This strong acid dissociates in the aqueous environment producing h 3 o hydronium ions. Humphreys where the atmospheric pressure. Explain why in terms of their vapor pressures and structural differences.
Part e the boiling point of a substance is defined as the temperature at which the vapor pressure is equal to the atmospheric pressure. Got a 250ml bottle for about 2. Diethyl ether is illegal in my country and i was trying to buy sulfuric acid and pure ethanol in a pharmacy to make it at home. Part d diethyl ether evaporates more quickly than ethanol.
Select all that apply dimethyl has weaker intermolecular forces ethanol has weaker intermolecular forces since it is not symmetrical dimethyl has stronger intermolecular forces dimethyl ether has a lower molar. Expert answer 100 12 ratings ethanol is ch3 ch2 o h diethyl ether is ch3 ch2 0 ch2 ch3 looking at those strucutures in ethanol the oxygen is bonded to a hydrogen we know that when hydrogen binds view the full answer.




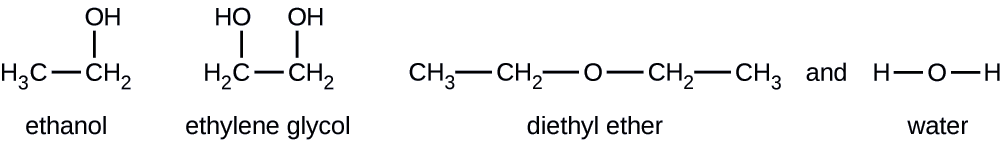





.jpg)



